Abstract
Background: Thrombopoietin receptor-agonists (TPO-RAs), eltrombopag (ELT) and romiplostim, are effective drugs licensed for relapsed/refractory ITP, leading to 70-80% response rates. As they stimulate the Mpl receptor on megakaryocytes, increasing platelet production, their effect is expected to be dependent on their continuous administration. However, durable remissions after TPO-RAs discontinuation have been described in 10-30% of patients (pts). This evidence pointed out a possible curative role for these agents, suggesting a mode of action that goes beyond the mere increase of platelet count, and interferes with the immune dysregulation underlying the disease, which worsens as ITP becomes chronic. The aim of this prospective study is to explore the role of ELT given for a defined period of time as second-line treatment in pts with newly diagnosed (ND) or persistent (P) ITP, thus interfering with the pathogenesis of the disease at an early stage, either offering a possible curative option for pts or a steroid-sparing drug as a bridge to chronic phase (CP).
Methods: Pts aged ≥18 years with ND or P ITP not responsive or in relapse after standard first-line therapy, with active disease (platelet count <30x109/L or steroids/IVIG dependence to maintain a platelet count ≥30x109/L and/or avoid bleeding) were included. The study was divided into a period of treatment (PT), during which pts received ELT at a starting dose of 50 mg/day, then adjusted according to platelet count, for 24 weeks, a period of tapering and discontinuation (PTD) (week 25 - 32), and a period of observation (PO) (week 33 - 52). Complete response (CR) was defined as a platelet count ≥100x109/L; response (R) as a platelet count ≥30x109/L and at least doubling of baseline count. Only pts in R or CR at the end of week 24 entered the PTD. The primary end-point was the proportion of pts who, being in remission at the end of PT, were able to taper down and discontinue ELT, maintaining the remission for all the PO, without requiring any concomitant therapies. Secondary objectives included the relationship between baseline TPO serum level and response to treatment, exploring modifications of immunological parameters across study phases and their correlation with responses.
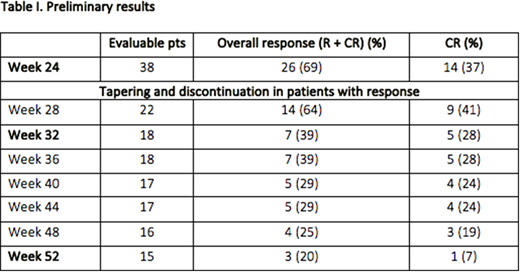
Results: Between 24/02/16 and 05/05/2018, 55 pts were enrolled by 10 GIMEMA Italian Centers. 3 pts didn't meet the inclusion criteria and 1 was excluded from study population because of protocol violation. Of the 51 evaluable pts, 31 (61%) were females. Median age was 65 years (range 21-90). 22 pts (44%) had ND ITP. Median baseline platelet count was 19x109/L (range 1-227). At the time of data cut-off, 10 pts were still in PT and 1 was not evaluable. Out of the remaining 40 pts, 2 were discontinued for reasons other than failure, and were excluded from response analysis. Of the 38 evaluable pts, at the end of 6 months of therapy 14 (37%) were in CR and 12 (32%) in R, with an ORR of 69%; 12 pts were non responders. All the 26 responders started the PTD. Of the 18 pts who completed the PTD, 7 maintained the response (ORR 39%), with 5 CR (28%) and 2 R (11%). At time of data cut-off, 11/26 pts have not completed yet the PO. 15/26 pts were evaluable at the end of the PO: 3 maintained the response (ORR 20%), with 1 CR and 2 R. 12 pts relapsed: 11 during the PTD and 1 during the PO. 17 pts (33%) reported a total of 58 adverse events (AEs), 8 pts (16%) reported a total of 11 grade ≥3 AEs. 4 AEs were considered treatment related, only 1 of them was grade ≥3 (deep vein thrombosis). 2 pts (3.9%) died during the study, for reasons not related to treatment.
Conclusions: These preliminary data confirm the previously reported efficacy of ELT in primary ITP, with an ORR of 69%. Almost 40% of pts maintained the response at the end of PTD, which is slightly higher than expected. These data suggest that the use of ELT at an earlier phase of the disease (ND or P ITP) is a more effective approach and that 6 months of therapy is a sufficient period to think about ELT tapering and discontinuation. Long-term remission was achieved in 20% of pts, but probably this data is biased by the fact that at the time of data cut-off only a small proportion of pts completed the PO, also considering that only 1 pt lost the response during the PO. For pts who don't maintain the response, ELT can still be a steroid-sparing agent used as a bridge to CP or other treatment options. This analysis is too early to define a correlation between the response and immunological parameters (including TPO levels).
Palandri:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Zaja:Amgen: Honoraria; Abbvie: Honoraria; Novartis: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Takeda: Honoraria; Janssen: Honoraria; Sandoz: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal